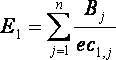
| Adisa Azapagic et al's environmental impact classification factors (EICF) |
This page is based heavily on the work of
Adisa Azapagic et al
as presented in the Appendix of Polymers, the Environment and Sustainable Development [1]
and Box A3 of the Appendix of Sustainable Development in Practice - Case Studies for Engineers and Scientists [2].
Azapagic et al use a problem-oriented approach to the definition of environmental impacts within the Inventory Analysis phase of Life Cycle Assessment under eight categories. The categories can be directly mapped to both ISO/TR 14047:2003(E) [3], the British Standard BS8905:2011 [4] and the European Environment Agency environmental impacts [5]:
| Azapagic et al | ISO/TR 14047:2003(E) | BS8905:2011 | European Environment Agency |
| Acidification Potential (AP) | Acidification | Acidification | Acidification |
| Aquatic Toxicity Potential (ATP) | Ecotoxicity | Ecotoxicity | Ecotoxicity |
| Eutrophication Potential (EP) | Eutrophication/Nitrification | Eutrophication | Eutrophication |
| Global Warming Potential (GWP) | Climate change | Global warming potential | Climate change and global warming |
| Human Toxicity Potential (HTP) | Human toxicity | Human toxicity | Human toxicity |
| Non-Renewable/Abiotic Resource Depletion (NRADP) | Depletion of abiotic/biotic resources | Resource depletion | |
| Ozone Depletion Potential (ODP) | Stratospheric ozone depletion | Stratospheric ozone depletion | Stratospheric ozone depletion |
| Photochemical Oxidants Creation Potential (POCP) | Photo-oxidant formation | Photochemical oxidation | Photochemical ozone formation (summer smog) |
| Land use |
NB: EICF for cork extraction and granulate processing are summarised on a separate page and are not included in the data below.
Non-Renewable/Abiotic Resource Depletion (NRADP) includes depletion of fossil fuels, metals and minerals. The total impact can be calculated using:
where Bj is the quantity (burden) of the resource used per functional unit (e.g. per kg of product for a chemical) and ec1,j represents the estimated total world reserves of that resource. Classification factors for NRADP are given in Table 1.
| Burden | Resource | Resource depletion (vs world reserves) | Units | References |
| Coal reserves | 87 200 | 109 tonnes | 1, 2 | |
| Coal proved reserves (2018) | 1055 | 109 tonnes | BP Stats 2019 review | |
| Coal production (2018) | 7.813 | 109 tonnes | WorldCoal | |
| Oil reserves | 124 | 109 tonnes | 1, 2 | |
| Oil proved reserves (2018) | 244 | 109 tonnes | BP Stats 2019 review | |
| Oil production (2018) | 4.474 | 109 tonnes | BP Stats 2019 review | |
| Gas reserves | 109 | 1012 m3 | 1, 2 | |
| Gas proved reserves (2018) | 197 | 1012 m3 | BP Stats 2019 review | |
| Gas production (2018) | 3.868 | 1012 m3 | BP Stats 2019 review |
Global Warming is caused by the atmosphere's ability to reflect some of the heat radiated from the earth's surface. This reflectivity is increased by the greenhouse gases (GHG) in the atmosphere. Increased emission of GHGs (CO2, N2O, CH4 and volatile organic compounds (VOCs)) will change the heat balance of the earth and result in a warmer climate over future decades. Global Warming Potential (GWP) is derived by summing the emissions of the GHG multiplied by their respective GWP factors, ec2,j. The GWP value is calculated in kg using:
where Bj represents the emission of greenhouse gas j. GWP factors, ec2,j, for different greenhouse gases are expressed relative to the GWP of CO2, which is therefore defined as unity. The values of the GWP depend on the time horizon over which the global warming effect is assessed. GWP factors for shorter times (20 years and 50 years) provide an indication of the short-term effects of greenhouse gases on the climate, while GWP values for longer periods (100 years and 500 years) are used to predict the cumulative effects of these gases on the global climate. Methane is removed from the atmosphere much more rapidly than CO2 so its short term effect is even greater than is suggested by the 100 year GWP [5]. The classification factors for GWP are given in Table 2a.
| Burden | Global Warming Potential (vs CO2 over 100 years) |
Lifetime in the atmosphere (years) [6] |
Percentage of 2000 emissions (in CO2e) [6] |
| CO2 (carbon dioxide) | 1 [1, 2] | 5-200 [6] or 50-200 [3] | 77% |
| CH4 (methane) | 11 [1] or 21 [2, 3] or 23 [6] | 10 [6] or 12±3 [3] | 14% |
| Nitrous oxide | 296 [6] or 310 [3] | 115 [6] or 120 [3] | 8% |
| Chlorinated hydrocarbons (HFCs) | 400 [1, 2] or 10-12000 [6] | 1-250 | 0.5% |
| Trichloroethane | 100 [7] | - | - |
| Chlorofluorocarbons (PFCs) | 5000 [1, 2] or >5500 [6] | >2500 | 0.2% |
| SF6 (sulphur hexafluoride) | 3200 | 22200 | 1% |
| Other volatile organic compounds | 11 [1, 2] | - | - |
The InterGovernmental Panel on Climate Change (IPCC) publication "Climate Change 2007: The Physical Science Basis - Summary for Policymakers" reported:
The amount of carbon dioxide released during the manufacture of different materials is given in Table 2b [8]. Timber contains stored carbon dioxide* from the atmosphere, so whilst some carbon dioxide is released during its harvesting and processing, "there is 8.3 kg of carbon dioxide [sic] absorbed during both the growth and milling process of timber", so no net carbon dioxide is produced. In the context of "renewable/bio-based" materials, note that the manufacture of fertiliser is ranked as the fifth of 123 UK production sectors for carbon intensity at 4.61 (units are percentage point change at £70/tonne carbon) and its use produces both methane and nitrous oxide emissions [6]. Those industries which are more carbon intensive than fertilisers are cement/lime and plaster at 9.00, electricity production and distribution at 16.07, refined petroleum at 23.44 and, finally, gas distribution at 25.36 [6].
| Material | CO2 (kg/kg material) |
| Aluminium | 27.50 kg |
| Copper | 8.5 kg (1.72-7.51kg [9]) |
| Glass | 1.30 kg |
| Iron | 1.75 kg |
| Lead | 2.50 kg |
| Lime | 1200 kg [10, 11] |
| Plastic | 3.40 - 11.00 kg |
| Rubber | 4.80 kg |
| Steel | 3.20 kg |
| Wood | n/a* |
There is a Table of embodied energy values at https://ecm-academics.plymouth.ac.uk/jsummerscales/MATS347/MATS347A9 NFETE.htm#energy.
“styrene is not expected to contribute to global warming” [Health Canada, 1993 and Kuhn et al, 2000]
The British Standard PAS 2050:2008 - Specification for the assessment of the life cycle greenhouse gas emissions of goods and services enables a consistent approach to measuring the embodied greenhouse gas emissions from products and services across their lifecycle, and is applicable to a wide range of sectors and product categories. It is expected that it will form the basis for an internationally agreed standard in this area.
Further reading: H Goosse, PY Barriat, W Lefebvre, MF Loutre and V Zunz, Introduction to climate dynamics and climate modeling, Online textbook, Université Catholique de Louvain, 2008.
Software: CCaLC is a carbon fooprinting tool for estimation of the life cycle greenhouse gas emissions throughout the whole supply chain. It uses the internationally accepted life cycle methodology defined by ISO 14044 and PAS2050, is claimed to be simple to use by non-experts and comes with comprehensive databases(including the Ecoinvent database).
Acidification is a consequence of acids (and other compounds which can be transformed into acids) being emitted to the atmosphere and subsequently deposited in surface soils and water. Increased acidity of these environments can result in negative consequences for coniferous trees (forest dieback) and the death of fish in addition to increased corrosion of manmade structures (buildings, vehicles etc.). The Vancouver Sun [12] has reported that anthropogenic CO2 emissions absorbed by the ocean may have pushed local waters through an acidity “tipping point” beyond which shellfish cannot survive: ten million dead scallops are the latest victims in the waters near Qualicum Beach.
Acidification Potential (AP) is based on the contributions of SO2, NOx, HCl, NH3 and HF to the potential acid deposition in the form of H+ (protons). The AP value is calculated in kg using:
where ec4,j represents the AP of gas j expressed relative to the value for SO2 and Bj is its emission in kg per functional unit. Classification factors for AP are given in Table 3.
| Burden | Acidification potential (vs SO2) | References |
| SO2 (sulphur dioxide) | 1 | 1, 2 |
| NOx (oxides of nitrogen) | 0.7 | 1, 2 |
| HCl (hydrogen chloride) | 0.88 | 1, 2 |
| HF (hydrogen fluoride) | 1.6 | 1, 2 |
| NH3 (ammonia) | 1.88 | 1, 2 |
Nutrient enrichment results from substances (primarily nitrogen and phosphorous from fertilisers) entering ecosystems and disturbing the normal biological balance. Some organisms may gain an unnatural advantage at the expense of other life forms. The principal consequence of nutrient enrichment is oxygen depletion, but normally low-nutrient habitats such as moorland can be damaged by such nutrient enrichment. The use of commercial inorganic fertilisers, in conjunction with increased livestock densities and the concentration of livestock production, has brought about a significant increase in the nutrient load on cultivated land. This material overload can result in runoff to watercourses leading in turn to eutrophication or contaminated water supply systems [5]. It has been known for decades that high levels of nitrogen deposition have the potential to change plant community composition and biodiversity. However, Emmett [13] has predicted substantial effects from lower, chronic levels of deposition.
Eutrophication Potential (EP) is defined as the potential of nutrients to cause over-fertilisation of water and soil which in turn can result in increased growth of biomass. The EP value is calculated in kg using:
where Bj is an emission of a species such as N, NOx, NH4+, PO43-, P and chemical oxygen demand (COD). The parameters ec5,j are measured relative to PO43-. Classification factors for EP are given in Table 4.
| Burden | Eutrophication Potential (vs PO43-) | References |
| Phosphates | 1 | 1, 2 |
| Nitrates | 0.42 | 1, 2 |
| Ammonia | 0.33 | 1, 2 |
| Oxides of nitrogen | 0.13 | 1, 2 |
| Chemical Oxygen Demand (COD) | 0.022 | 1, 2 |
Sundqvist [14] references eutrophicating substances to Chemical Oxygen Demand (DOD) with 1 kg of NO3- equal to 4.4 kg COD, 1 kg of NOx equal to 6 kg of COD, 1 kg of NH3 equal to 16 kg COD and 1 kg phosphorus equal to 140 kg COD.
Kurtz et al [15] found that the survival of larvae in six Lepidoptera species decreased by at least one-third, without clear differences between sorrel- and grass-feeding species, when nitrogen fertiliser was applied to the host plants at rates typically used in current farming practice.
Photochemical Oxidant Formation results from the degradation of the oxides of nitrogen (NOx) and volatile organic compounds (VOCs) in the presence of light. Excess ozone can lead to damaged plant leaf surfaces, discolouration, reduced photosynthetic function and ultimately death of the leaf and finally the whole plant. In animals, it can lead to severe respiratory problems and eye irritation. Photochemical Oxidants Creation Potential (POCP) is related to the potential for VOCs and oxides of nitrogen to generate photochemical or summer smog. It is usually expressed relative to the POCP classification factor for ethylene. The POCP value is calculated in kg using:
where Bj is the emission of the species participating in the formation of summer smog and ec6,j is the classification factor for photochemical oxidation formation. Classification factors for ATP are given in Table 5:
| Burden | Photochemical Smog (vs Ethene) | References |
| Ethene (a.k.a. Ethylene) | 1 | 1, 2 |
| Methane | 0.006-0.007 | 1, 2, 14 |
| Carbon monoxide | 0.030 | 14 |
| Styrene | 0.142 | 16 |
| Other hydrocarbons (except methane) | 0.416 | 1, 2 |
| Non-methane volatile organic compounds (NMVOC) | 0.416 | 14 |
| Aldehydes | 0.443 | 1, 2 |
| Other volatile organic compounds | 0.007 | 1, 2 |
Ozone is formed and depleted naturally in the earth's stratosphere (between 15-40 km above the earth's surface). Halocarbon compounds are persistent synthetic halogen containing organic molecules that can reach the stratosphere leading to more rapid depletion of the ozone. As the ozone in the stratosphere is reduced more of the ultraviolet rays in sunlight can reach the earth's surface where they can cause skin cancer and reduced crop yields. Ozone Depletion Potential (ODP) indicates the potential for emissions of chlorofluorocarbon (CFC) compounds and other halogenated hydrocarbons to deplete the ozone layer. Chlorofluorocarbons are now of minor importance since they were banned by the Montreal Protocol [17].
Fertiliser-induced N2O emissions are now the single most important driver of stratospheric ozone depletion on a global scale [18]. Extensive farming may now be seeing decreasing yields, and combined with competition for land use between food, fuel and bio-based feedstocks, there is a need for a thorough evaluation of bio-based materials at local, regional, national and global scales [17, 19, 20]. The ODP value is calculated in kg using:
where Bj is the emission of an ozone-depleting gas j. The ODP factors ec3,jare expressed relative to the ODP of trichlorofluoromethane (CFC-11) [21]. Classification factors for ODP are given in Table 6.
| Burden | Ozone Depletion Potential (vs CFC-11) | References |
| Trichlorofluoromethane (CFC-11) | 1 | 1, 2 |
| Chlorinated hydrocarbons | 0.5 | 1, 2 |
| Chlorofluorocarbons | 0.4 | 1, 2 |
| Other volatile organic compounds | 0.005 | 1, 2 |
Human and Eco-Toxicity result from persistent chemicals reaching undesirable concentrations in each of the three elements of the environment (air, soil and water) leading to damage to humans, animals and eco-systems. The modelling of toxicity in LCA is complicated by the complex chemicals involved and their potential interactions. Azapagic [1, 2] states that “Toxicity estimations in LCA are notoriously unreliable and difficult to quantify”.
While natural products are often assumed to be benign, inhalation of lignocellulosic dust from processing of plant fibres in an inadequately ventilated working environment can lead to narrowing of the airways (byssinosis).
Human Toxicity Potential (HTP) takes account of releases of materials toxic to humans in three distinct media - air (A), water (W) and soil (S). The HTP value is calculated in kg using:
where ec7,jA, ec7,jW and ec7,jS are human toxicological classification factors for substances emitted to air, water and soil respectively and BjA, BjW and BjS represent the respective emissions of the different toxins to each of the media. The toxicological factors are calculated using scientific estimates for the acceptable daily intake or tolerable daily intake of the toxic substances. The human toxicological factors are still at an early stage of development so that HTP can only be taken as an indication and not as an absolute measure of the toxicity potential. Classification factors for HTP are given in Table 7.
| Burden | Human Toxicity Potential | References |
| CO (carbon monoxide) | 0.012 | 1, 2 |
| NOx (oxides of nitrogen) | 0.78 | 1, 2 |
| SO2 (sulphur dioxide) | 1.2 | 1, 2 |
| Hydrocarbons (excluding methane) | 1.7 | 1, 2 |
| Chlorinated hydrocarbons | 0.98 | 1, 2 |
| Chlorofluorocarbons | 0.022 | 1, 2 |
| As (arsenic vapour) | 4700 | 1, 2 |
| Hg (mercury vapour) | 120 | 1, 2 |
| F2 (fluorine) | 0.48 | 1, 2 |
| HF (hydrogen fluoride) | 0.48 | 1, 2 |
| NH3 (ammonia) | 0.0017-0.020 | 1, 2 |
| As (arsenic as solids) | 1.4 | 1, 2 |
| Cr (chromium) | 0.57 | 1, 2 |
| Cu (copper) | 0.02 | 1, 2 |
| Fe (iron) | 0.0036 | 1, 2 |
| Hg (mercury as liquid) | 4.7 | 1, 2 |
| Ni (nickel) | 0.057 | 1, 2 |
| Pb (lead) | 0.79 | 1, 2 |
| Zn (zinc) | 0.0029 | 1, 2 |
| Fluorides | 0.041 | 1, 2 |
| Nitrates | 0.00078 | 1, 2 |
| Phosphates | 0.00004 | 1, 2 |
| Chlorinated solvents and compounds | 0.29 | 1, 2 |
| Cyanides | 0.057 | 1, 2 |
| Pesticides | 0.14 | 1, 2 |
| Phenols | 0.048 | 1, 2 |
Styrene has an odour threshold 0.08 ppm (parts per million) [22] to 0.32 ppm [23]. Permitted levels in the workplace range from 10 ppm (new build facilities in Sweden) to 100 ppm (e.g. UK) for time weighted average exposure. The NIOSH (National Institute for Occupational Safety and Health) IDLH (Immediately Dangerous to Life or Health) level for styrene is 700 ppm [24] based on acute inhalation toxicity data in humans [25, 26].
In the United States, the National Toxicology Program 12th Report on Carcinogens [American Composites Manufacturers Association - Regulatory Bulletin - 23 July 2008] has recommended that styrene be listed as "reasonably anticipated to be a human carcinogen".
Aquatic Toxicity Potential (ATP) has a value calculated in m3 using:
where ec8,jA represents the toxicity classification factors of different aquatic toxic substances and BjA is their respective emissions to the aquatic ecosystem. ATP is based on the maximum tolerable concentrations of different toxic substances in water by aquatic organisms. As with HTP, the classification factors for ATP are still developing and hence should only be used as indicators of potential toxicity. Classification factors for ATP are given in Table 8.
| Burden | Aquatic toxicology (m3 x 1012/g ) | Aquatic toxicology (pg/m3 ) | References |
| As (arsenic) | 0.181 | 5.52 | 1, 2 |
| Cr (chromium) | 0.907 | 1.10 | 1, 2 |
| Cu (copper) | 1.810 | 0.55 | 1, 2 |
| Hg (mercury) | 454 | 0.0022 | 1, 2 |
| Ni (nickel) | 0.299 | 3.34 | 1, 2 |
| Pb (lead) | 1.810 | 0.55 | 1, 2 |
| Zn (zinc) | 0.345 | 2.90 | 1, 2 |
| Oils and greases | 0.0454 | 22.0 | 1, 2 |
| Chlorinated solvents and compounds | 0.0544 | 18.4 | 1, 2 |
| Pesticides | 1.180 | 0.85 | 1, 2 |
| Phenols | 5.350 | 0.19 | 1, 2 |
Azapagic [27] has recently stated that "Toxicity estimations in LCA are notoriously unreliable and difficult". While the dilution volume method above does represent an approach previously used , an updated methodology has now been developed (driven by CML in the Netherlands). The method now predominantly used to calculate eco-toxicity (including aquatic ecotoxicity) is based on 1,4-dichlorobenzene equivalence (with units of kg 1,4-DB eq.). Eco-toxicity potential (ETP) is calculated for all three environmental media and comprises five ETPn indicators:
where n (in the range 1-5) represents freshwater aquatic toxicity, marine aquatic toxicity, freshwater sediment toxicity, marine sediment toxicity and terrestrial ecotoxicity respectively. ETPi,j represents the ecotoxicity classification factor for toxic substance j in the compartment i (air, water, soil) and Bi,j is the emission of substance j to compartment i. ETP is based on the maximum tolerable concentrations of different toxic substances in the environment by different organisms.
References